New Diabetes Drug Delays Type 1 in Adults
New Diabetes Drug Delays Type 1 in Adults for First Time in UK
What happened
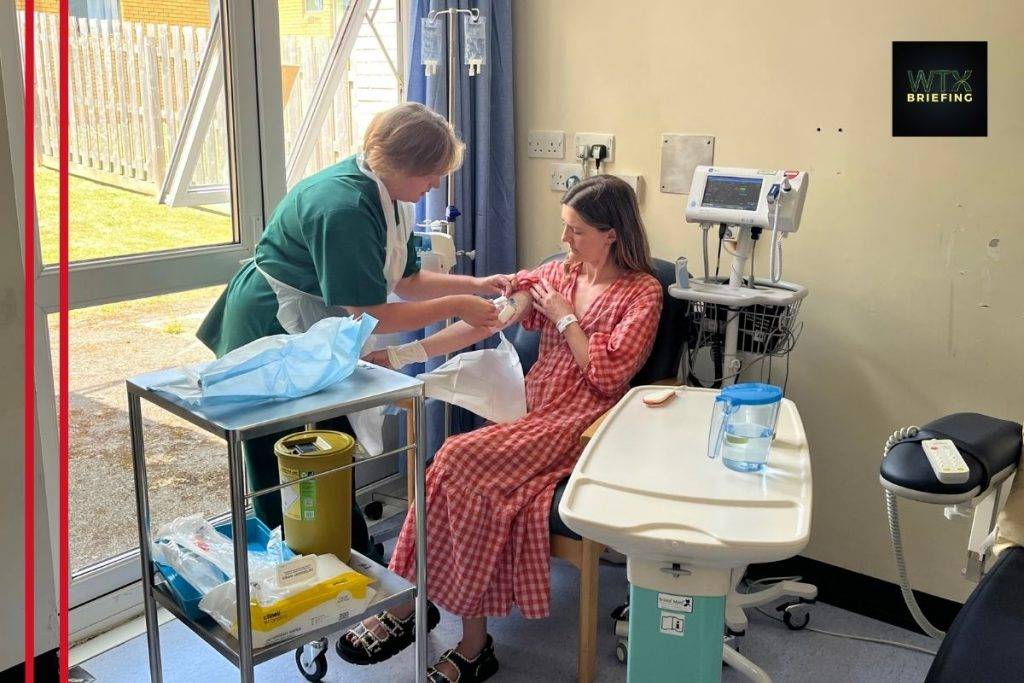
Hannah Robinson, a 36‑year‑old dentist and mother of two from Devon, has become the first UK adult to receive the immune‑modulating drug teplizumab. Administered at Royal Devon University Hospital NHS Foundation Trust, this treatment is given in early-stage type 1 diabetes to delay the body’s insulin dependency by about three years, delaying pancreatic cell loss.
Why it matters
This marks a major breakthrough in managing type 1 diabetes in adults. Unlike traditional insulin therapy, teplizumab tackles the underlying autoimmune attack, potentially transforming treatment approaches. If successful, it could lead to screening programmes, early interventions, and widespread NHS adoption, offering “precious extra years insulin-free” for many.
Reaction
Robinson described the treatment as offering freedom and control, not being defined by her condition. Dr Nick Thomas, a diabetes consultant, hailed it as “a really exciting shift” in disease management. Diabetes UK’s Dr Lucy Chambers stressed the need for license approval, screening, and NHS readiness to expand use.
What next
Experts aim to secure a UK licence for teplizumab, develop national early‑risk screening, and build NHS capacity for wider use. The Royal Devon and the University of Exeter are working to identify at‑risk patients using genetic and autoantibody testing. If approved, teplizumab could become the first immunotherapy that alters the course of type 1 diabetes in adults.